Many solutions contain one component, called the solvent, in which other components, called solutes, are dissolved. An aqueous solution is one for which the solvent is water. The concentration of a solution is a measure of the relative amount of solute in a given amount of solution. Concentrations may be measured using various units, with one very useful unit being molarity, defined as the number of moles of solute per liter of solution. The solute concentration of a solution may be decreased by adding solvent, a process referred to as dilution.
The dilution equation is a simple relation between concentrations and volumes of a solution before and after dilution. A disadvantage of describing formulas as w/v (%) is that the description says nothing about the actual concentration of molecules in solution. What if we want equal amounts of two chemicals to be mixed together, so that for each molecule of substance #1 there is a single molecule of substance #2?
The same amount in grams will likely not contain the same number of molecules of each substance. For example, you may work with a chemical that can be in one of several forms of hydration. Calcium chloride can be purchased as a dry chemical in anhydrous form, so that what you weigh out is nearly all pure calcium chloride. On the other hand you may have a stock of dry chemical that is hydrated with seven water molecules per molecule of calcium chloride. The same mass of this chemical will contain fewer molecules of calcium chloride. As long as the molecular weight is known, we can describe a solution in the form of moles per liter, or simply molar .
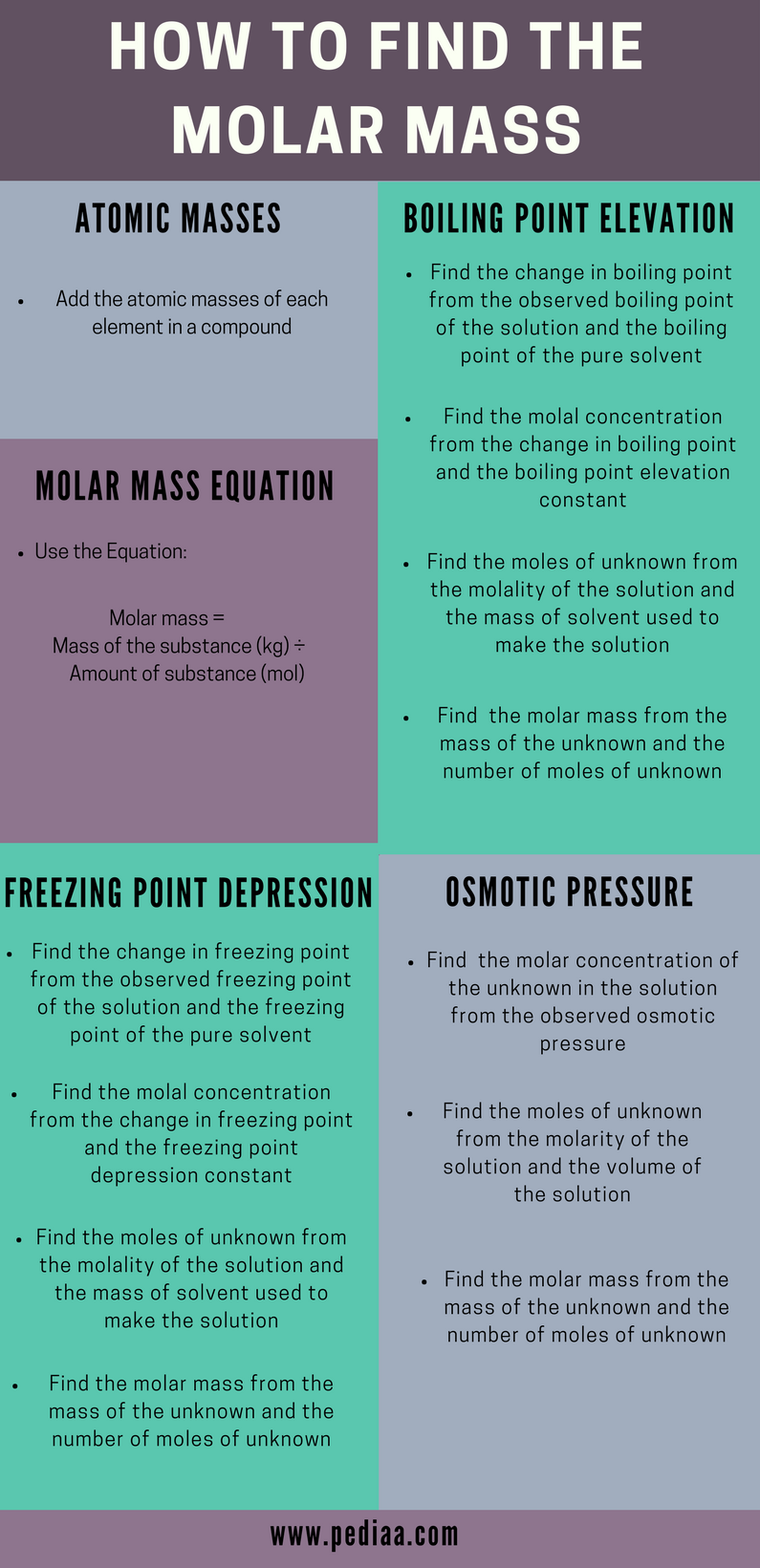
As with w/v solutions, we weigh out a specific amount of chemical when making a molar solution. Unlike w/v solutions, the amount to weigh depends on the molecular weight (m.w.) of the substance in grams per mole (g/mol). In order to calculate the desired mass of solute you will need to know the formula weight.
Formula weights are usually printed on the label and identified by the abbreviation f.w. Formula weight is the mass of material in grams that contains one mole of substance, and may include inert materials and/or the mass of water molecules in the case of hydrated compounds. For pure compounds the formula weight is the molecular weight of the substance and may be identified as such. To calculate molarity, divide the number of moles of solute by the volume of the solution in liters. Once you have the molar mass, multiply the number of grams of solute by 1 over the molar mass to convert the grams into moles. Finally, divide the number of moles by the volume of the solution to get the molarity.
To calculate the molarity of a solution, the number of moles of solute must be divided by the total liters of solution produced. Suppose that someone has already worked out the details, so all that you have to do is read a formula and make a solution. We can usually assume that a solution is to be aqueous unless stated otherwise. What about the concentration of the substance to be added? Common ways of describing the concentrations of solutions are weight-in-weight, weight-in-volume, volume-in-volume, and molarity. Less commonly used descriptions include normality and molality.
A quantity of solute is measured out, mixed with solvent, and the volume is brought to some final quantity after the solute is completely dissolved. That is, solutions are typically prepared volumetrically. Because solutes add volume to a quantity of solvent, this method of preparation of solutions is necessary to ensure that an exact desired concentration is obtained.
Figure 4.6 "Preparation of a Solution of Known Concentration Using a Solid Solute" illustrates this procedure for a solution of cobalt chloride dihydrate in ethanol. Note that the volume of the solvent is not specified. Because the solute occupies space in the solution, the volume of the solvent needed is almost always less than the desired volume of solution.
For example, if the desired volume were 1.00 L, it would be incorrect to add 1.00 L of water to 342 g of sucrose because that would produce more than 1.00 L of solution. As shown in Figure 4.7 "Preparation of 250 mL of a Solution of (NH", for some substances this effect can be significant, especially for concentrated solutions. An aqueous solution consists of at least two components, the solvent and the solute . Usually one wants to keep track of the amount of the solute dissolved in the solution.
One could do by keeping track of the concentration by determining the mass of each component, but it is usually easier to measure liquids by volume instead of mass. To do this measure called molarity is commonly used. Molarity is defined as the number of moles of solute divided by the volume of the solution in liters. As an example, equal volumes of gaseous hydrogen and nitrogen contain the same number of atoms when they are at the same temperature and pressure, and observe ideal gas behavior. In practice, real gases show small deviations from the ideal behavior and the law holds only approximately, but is still a useful approximation for scientists.
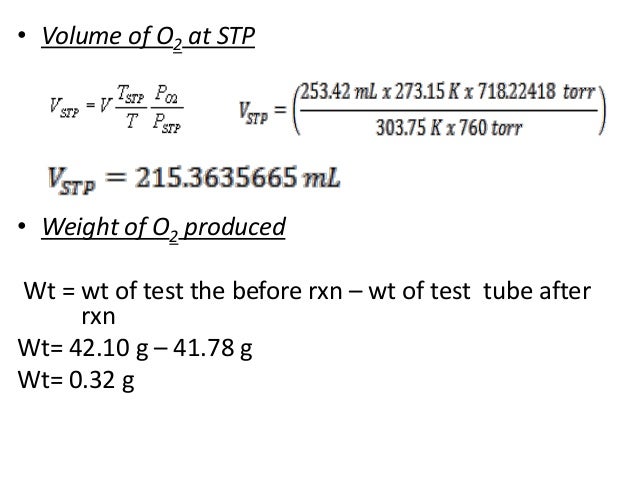
Again consider the reaction in equation 3 above. First convert this volume into mass using density (g/mL), then convert grams to moles using the molecular weight. Again, include units and set up your calculation so that milliliters and grams cancel in the calculation leaving an answer that has units of moles. Molality is an intensive property of solutions, and it is calculated as the moles of a solute divided by the kilograms of the solvent. Unlike molarity, which depends on the volume of the solution, molality depends only on the mass of the solvent. Since volume is subject to variation due to temperature and pressure, molarity also varies by temperature and pressure.
In some cases, using weight is an advantage because mass does not vary with ambient conditions. For example, molality is used when working with a range of temperatures. Nevertheless, related experiments with some inorganic substances showed seeming exceptions to the law. This apparent contradiction was finally resolved by Stanislao Cannizzaro, as announced at Karlsruhe Congress in 1860, four years after Avogadro's death.
He explained that these exceptions were due to molecular dissociations at certain temperatures, and that Avogadro's law determined not only molecular masses, but atomic masses as well. The concentration of a substance is the quantity of solute present in a given quantity of solution. Concentrations are usually expressed as molarity, the number of moles of solute in 1 L of solution.
It is important to note that the molarity is defined as moles of solute per liter of solution, not moles of solute per liter of solvent. This is because when you add a substance, perhaps a salt, to some volume of water, the volume of the resulting solution will be different than the original volume in some unpredictable way. To get around this problem chemists commonly make up their solutions in volumetric flasks. These are flasks that have a long neck with an etched line indicating the volume. The solute is added to the flask first and then water is added until the solution reaches the mark.
The flasks have very good calibration so volumes are commonly known to at least four significant figures. Both terms are used to express the concentration of a solution, but there is a significant difference between them. While molarity describes the amount of substance per unit volume of solution, molality defines the concentration as the amount of substance per unit mass of the solvent. In other words, molality is the number of moles of solute per kilogram of solvent .
The units of molar concentration are moles per cubic decimeter. They are noted as mol/dm³ as well as M (pronounced "molar"). In many older books or articles, you can find different units of molar solutions – moles per liter (mol/l). Remember that one cubic decimeter equals to one liter, so these two notations express the same numeric values.
Most typically you will find it as grams per milliliter or g/mL. If you know the molecular formula of the substance, you can then calculate the molarity of the solution because grams can be converted to mol and mL can be converted to liters. Finally, you can find the number of mol in a certain volume of said substance. Concentrations are often reported on a mass-to-mass (m/m) basis or on a mass-to-volume (m/v) basis, particularly in clinical laboratories and engineering applications.
Each measurement can be expressed as a percentage by multiplying the ratio by 100; the result is reported as percent m/m or percent m/v. For aqueous solutions at 20°C, 1 ppm corresponds to 1 μg per milliliter, and 1 ppb corresponds to 1 ng per milliliter. These concentrations and their units are summarized in Table 4.1 "Common Units of Concentration".
Is the volume occupied by one mole of a chemical element or a chemical compound. It can be calculated by dividing the molar mass by mass density (ρ). Molar gas volume is one mole of any gas at a specific temperature and pressure has a fixed volume. Perhaps the easiest way to describe a solution is in terms of weight-in-weight (w/w). The weight of the solute relative to the weight of the final solution is described as a percentage. For example, suppose you have a dye that is soluble in alcohol.
Rather than write the instructions, "take 3 grams dye and mix with 97 grams absolute alcohol," you can describe the solutions simply as 3% dye in absolute alcohol. The formula applies to any volume of solution that might be required. Three grams dye plus 97 grams alcohol will have final weight of 100 grams, so the dye winds up being 3% of the final weight. Note that the final weight is not necessarily equal to the final volume. Aqueous weight-in-weight solutions are the easiest to prepare.
Since 1 milliliter of water weighs one gram, we can measure a volume instead of weighing the solvent. A very common use of w/w formulas is with media for the culture of bacteria. Such media come in granular or powdered form, often contain agar, and often require heat in order to dissolve the components. Microbiological media, especially when they contain agar, are difficult to transfer from one vessel to another without leaving material behind. They coat the surfaces of glassware, making quite a mess. Using a w/w formula the media and water can be mixed, heated, then sterilized, all in a single container.
Very little material is wasted and there is less of a mess. The atomic weight of a substance is an assigned number which allows comparison of the relative masses of the different elements. By definition, one atom of oxygen is assigned a "weight" of 16, and the atomic weights of the other elements are determined in relation to that of oxygen. In a molecule, i.e., a substance containing two or more different atoms, the molecular weight is equal to the sum of the atomic weights of the individual atoms.
For example, the molecular weight of water is 18 ( + 16). Molecules in a litre or even a cubic centimetre is enormous, it has become common practice to use what are called molar, rather than molecular, quantities. A mole is the gram-molecular weight of a substance and, therefore, also Avogadro's number of molecules (6.02 × 1023). Concentration in moles per litre (i.e., molarity) is usually designated by the letter M. We then convert the number of moles of solute to the corresponding mass of solute needed.
Avogadro was an Italian Physicist who first described the Avogadro constant as a hypothesis in 1811. He was trying to understand why in chemical reactions involving gases the observation that equal volumes of different gases had the same number of moles. This was found true even when the masses were very different.
The idea that a mole of any substance has exactly the same number of atoms no matter what the substance is made of was explained by Avogadro and his name has stuck to his number ever since. Provided some additional information is known, one value can be deduced from the other using the equations below. You can get your moles by taking the molar mass of each of the elements in the solute and adding them together. Do it; the answer is in moles because the grams cancelled out.Then, go ahead and do your formula. Now that you have the molar mass of the solute, you need to multiply the number of grams of solute in the solution by a conversion factor of 1 mole over the formula weight of the solute.
This will give you the number of moles of the solute for this equation. To calculate molarity, you can start with moles and volume, mass and volume, or moles and milliliters. Plugging these variables into the basic formula for calculating molarity will give you the correct answer.
A one percent solution is defined as 1 gram of solute per 100 milliliters final volume. For example, 1 gram of sodium chloride, brought to a final volume of 100 ml with distilled water, is a 1% NaCl solution. To help recall the definition of a 1% solution, remember that one gram is the mass of one milliliter of water. The mass of a solute that is needed in order to make a 1% solution is 1% of the mass of pure water of the desired final volume.
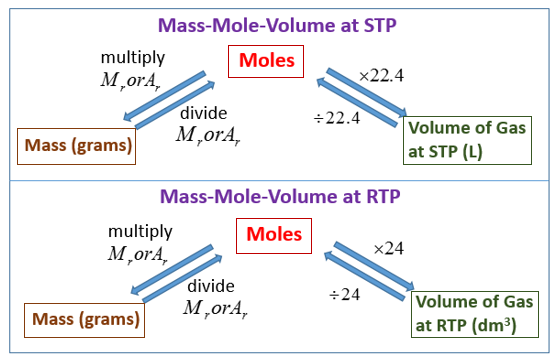
Examples of 100% solutions are 1000 grams in 1000 milliliters or 1 gram in 1 milliliter. We saw in Moles, mass and gas calculations that for practical reasons, gas is usually measured in volume as opposed to mass which is used for solids. When we fix the temperature and pressure, at STP or RTP, the volume that one mole of an ideal gas occupies is also fixed, or constant.
Where Z is the gas compressibility factor, which is a useful thermodynamic property for modifying the ideal gas law to account for behavior of real gases. The above equation is basically a simple equation of state . So you are not confused with similar chemical terms, keep in mind that molarity means exactly the same as molar concentration . Molarity expresses the concentration of a solution. It is defined as the number of moles of a substance or solute, dissolved per liter of solution (not per liter of solvent!). The relative formula mass of a compound is calculated by adding together the relative atomic mass values for all the atoms in its formula.
Moles are units used to measure substance amount. One mole of water has a mass of 18 grams and volume of 18 milliliters or 0.018 liters.How much is a mole of water? A mole of water is Avogadro's number of water molecules. Avogadro's number is so large that it can be difficult to imagine its size. Finding the mass and volume of one mole of water is a great way to relate the units to something familiar.








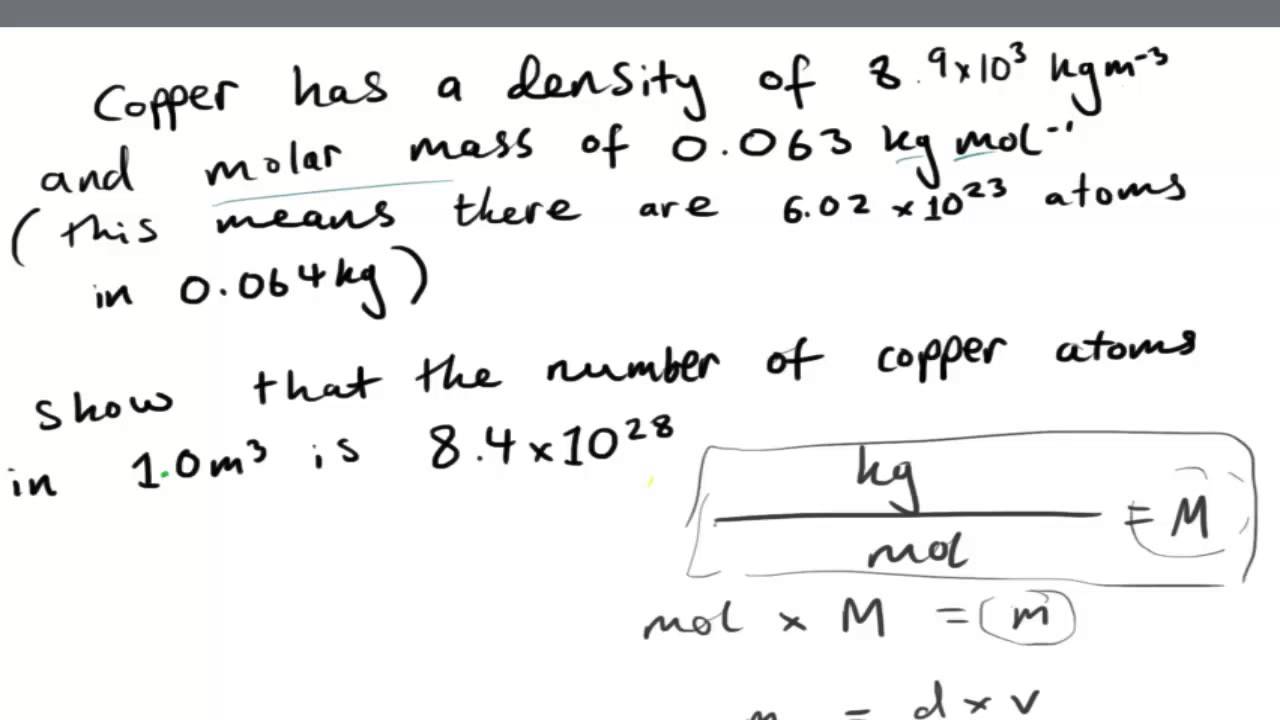















No comments:
Post a Comment
Note: Only a member of this blog may post a comment.